ionization energy equation
For the Hydrogen Atom ionization from the ground state where ni 1 ΔEizn 218 1018J 1 2 1 12 218 1018Joules to remove the electron from n 1. So the general ionization equation isA energy A.
 |
| Ionization Energy Basic Introduction Youtube |
In this article Ionization energy examples with their detailed explanations are derived.
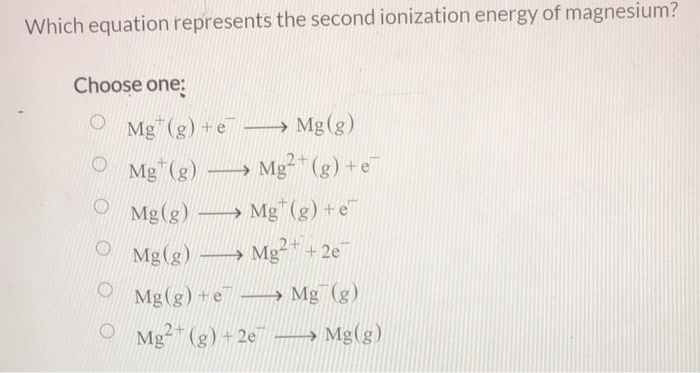
. Roughly speaking the closer the outermost electrons are to the nucleus of the atom. That means the total number of electrons in its atom are 15. 2 If an atom or molecule gains an electron it becomes negatively charged an. What is the Ionization Energy Formula.
The energies of electrons in molecular orbitals can be observed directly by measuring the ionization energy. Ionization energy is positive for neutral atoms meaning that the ionization is an endothermic process. The basic equation for ionization energy is. The atomic number of Phosphorus is 15.
The ionization energy is the quantity of energy that an isolated gaseous atom in the ground electronic state must absorb to discharge an electron resulting in a cation. Ionization potential for hydrogen can be calculated using the following equation. The first ionisation energy of. The third ionization energy can be represented by the following equation.
The n th ionization energy refers to the amount of. Ionization energy is the energy required to remove the electron completely from the atom so as to convert it to a positive ion. In respect of Al the ionization energies first second and third are 578 1817 and 2745. Ionisation energy The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state.
The first ionization energy IE 1 is the energy needed to remove the first electron from the atom in gaseous state. 1 Ionization is the process by which ions are formed by gain or loss of an electron from an atom or molecule. Now we said that the ionization energy is the energy difference between the energy of the electron in the initial orbit and the energy of the electron in the infinite orbit. E hcRH 1n 2 where E is energy of the electron or the amount of energy it takes to remove the electron.
Na 2 g energy Na 3 g e-The energy required to form a Na 3 ion in the gas phase is the sum of the first. Answer 1- The total energy to bring about the change IE1 IE2 IE3 578 1817 2745 5140. The meaning of higher ionization energy means facing the difficulty to subtract any electron from. Basic Equation of Ionization Energy.
Ionization Energy is the amount of energy needed to REMOVE an electron from an atom in the gas phase. 1 H g. The ionization energy is the amount of energy required to take an electron from its ground state or most stable state to infinity. Xg Xg e-The amount of energy necessary changes each time an electron is let go since it becomes.
The ionization energy of an element is the minimum energy required to remove an electron from the valence shell of an isolated gaseous atom to form an ion 1. H IE H e IE 135984 eV The ionization energy associated with removal of the first electron is most commonly used. This is the energy required to remove an electron in this case from a. The second ionization energy IE 2 is the energy needed to.
Thus if we assume 0 eV as the reference at. Ionization energy graph of Phosphorus. The values of ten ionization energies of.
 |
| 1 Ionization Energy Theory Iet Equations In Condensed Matter Download Scientific Diagram |
 |
| Ionization Energy Equations Youtube |
 |
| Ionization Energy Zirconium Zirconium Ppt Video Online Download |
 |
| Why Does This Equation Not Give Me The Ionization Energy Of Lithium Physics Forums |
 |
| Ionisation Energy Definitions The First Ionisation Energy Is The Energy Required To Remove The First Or Outermost Electron From Each Atom In A Mole Ppt Download |
Posting Komentar untuk "ionization energy equation"